Services
The Ross Fluorescence Imaging Center is an open access light microscopy core facility at the Johs Hopkins University East Baltimore campus.
Available services include:
- Advice and consent on sample preparation desired scientific approach
- Our instruments, experimental methods and applications include, -- http://confocal.jhu.edu/current-equipment/
- FISHscope = Olympus IX83 inverted microscope -- see http://confocal.jhu.edu/current-equipment/fishscope
- Olympus FV3000RS Confocal Microscope, 7 lasers, 6 detectors, see http://confocal.jhu.edu/current-equipment/fv3000
FV3000RS was funded by NIH shared instrumentation grant 1S10OD025244-01 to Prof. Brian O'Rourke, with Prof. Mark Donowitz, co-P.I. - most projects are through our NIDDK P30 Center.
Please cite the grant number in your acknowledgement section!
- Leica SP8 inverted confocal microscope - owned by Anesthesiology & Critical Care Medicine (ACCM, a division of JHU Department of Medicine), managed by us. Please use JHU iLab Organizer for scheduling,
- Live cell and tissue imaging using an Olympus FV1000 MP mulitphoton excitation fluorescence NDD4 microscope, with ratiometric ionic probes (Indo-1 or SNARF), CFP/YFP, and "RGB" filter sets.
- (legacy) Andor Revolution X1 spinning disk confocal microscope with TuCam image splitter and dual EMCCDs.http://confocal.jhu.edu/current-equipment/andor-spinning-disk
- Keyence BZ-X700 "box" microscope (no eyepieces), can handle many different specimen formats.
- Measurements of intracellular pH (potentially other ions) on cell populations using PTI’s RatioMaster and QuantMaster spectrofluorometic systems, equipped with temperature controlled perfusion chambers.
- Access to MetaMorph and Volocity software for advanced image analysis, deconvolution, tracking, etc.
- Comprehensive instruction on all facility equipment
- Individualized sample preparation is not available at his time
- Please click on the following link for a complete list of equipment, location and scheduling: Current Equipment
20180711Wed: Our two main instruments are confocal microscopes. With the arrival of our Olympus FV3000RS confocal microscope, I am posting my recommended XY pixel and Z step size to get optimal image quality from our confocals + GPU deconvolution (SP8 -> HyVolution2 or FV3000RS w/Olympus GPU deconvolution).
McNamara 20180711W Recommended Confocal Microscope Pixel Sizes
XY resolution: d = 0.6 * Lambda / NA … pixel size = d/3
Z resolution = 3 * XY resolution
Leica SP8 = ACCM Confocal Microscope (iLab Organizer)
* 405, 488, 552, 638 nm lasers
* HyD1, PMT2, HyD3 ... I normally use the HyD's in photon counting mode (16-bit acquisition), 10 line accumulation (more or less if needed or usable, respectively). Emission band at least 10 nm from laser line (more if close to coverglass or slide).
Objective lens XY Pixel size Z step WD (working distance)
20x / 0.75NA dry 120 nm 360 nm 0.62 mm
63x / 1.40 NA oil 50 nm 150 nm 0.14 mm
* On our SP8, I suggest 600Hz for maximum flexibility of Zoom (0.75 to 10+), and higher scan speed if possible (SP8 max is 1800 Hz, zoom 7.5 or more). Faster scan speed and more line accumulation => generally better fluorophore photophysics than same 'total dwell time'. That is, 600 Hz * 10 line accumulation
Olympus FV3000RS confocal Microscope
* 405nm, 445nm, 488nm, 514nm, 561nm, 640nm and 730nm lasers.
* 4 GaAsP high sensitivity, TRU Spectral detectors
* 2 external GaAs red-shifted high sensitivity detectors
Standard (dry or oil) objective lenses
Objective lens mag NA XY Pixel size Z step WD
PLAN APO 2X 0.08 1250 nm 3750 nm 6.2 mm
UPLAN S-APO 10X 0.4 250 nm 750 nm 3.1 mm
U PLAN S-APO 20X 0.75 120 nm 360 nm 0.6 mm
U PLAN S-APO 60X 1.35 oil 50 nm 150 nm 0.15 mm
Silicone Oil objective lenses
Objective lens XY Pixel size Z step WD
UPLSAPO N 30X 1.05 SI 100 nm 300 nm 0.8 mm
UPLSAPO 40x 1.25 SI 80 nm 240 nm 0.3 mm
UPLSAPO 100x 1.35 SI 50 nm 150 nm 0.2 mm
Why these pixel and Z settings? Yes.
Some answers might (or not) be in 'my' CPHG Unit (which awesomely is open access) at https://currentprotocols.onlinelibrary.wiley.com/doi/abs/10.1002/cphg.42
If you are like Sammy Hager, and cannot drive 1.4NA with 50nmXY and 150nmZ ( https://www.youtube.com/watch?v=RvV3nn_de2k ) that's nice. But hopefully you have a better reason than the microscope company default settings of 512x512 pixels and 400 Hz.
NIRF: Near Infrared Fluorescence
Our new Olympus FV3000RS confocal microscope will have a 730nm laser and two NIR GaAs PMTs (Gallium Arsenide photomultiplier tubes):
730nm lasers.
* 4 GaAsP high sensitivity, TRU Spectral detectors
* 2 external GaAs red-shifted high sensitivity detectors
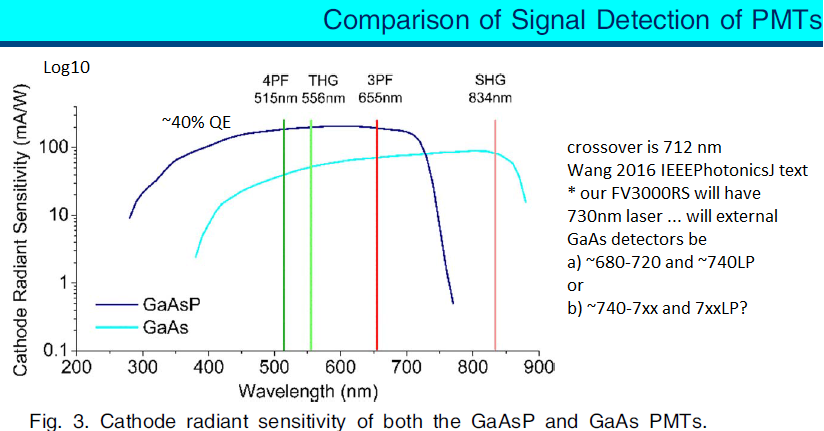
Figure from:
Wang Y, Wang K, Wen W, Qiu P, Wang K 2016 Comparison of Signal Detection of GaAsP and GaAs PMTs for Multiphoton Microscopy at the 1700-nm window. IEEE Photonics J 8: 6803406
https://ieeexplore.ieee.org/document/7471455
We are not the only light microscopy core facility at Johns Hopkins University. We will try to keep the links below up to date - if you find a non-functional link, or would like us to add a link, please contact George McNamara, gmcnamara@jhmi.edu with information.
We note there are several Zeiss LSM 880 or 980 AiryScan or AiryScan2 confocal microscopes at the East Baltimore campus, including:
* ICE = Institute for Cellular Engineering (Miller Bldg)
* MicFac (Physiology Bldg)
* Wilmer Eye Institute (see Wilmer web page)
? (maybe) Neuroscience (PCTB?)
We note that AiryScan and AiryScan2 data would likely benefit from SVI.nl Huygens deconvolution for AiryScan, see https://svi.nl/Huygens-Array-Detector-Software which may compute better data than what Zeiss produces (Huygens needs the raw data file format from the Zeiss microscopes). We routinely encourage our confocal microscope users to use deconvolution, either Leica SP8 --> Hyugens Essential ("HyVolution2" in the Leica SP8 LAS X software), or FV3000RS confocal and FISHscope widefield - or our other Z-series capable microscopes -- data to the Olympus cellSens deconvolution software (which was purchased on the FV3000RS grant, so use should have that grant number in acknowledgement).
Our current user base -- mostly G.I., ACCM, and cardiology, with many other School of Medicine Departments, and Dept of Medicine Divisions, represented. We do our best to keep informed of our users needs (mostly money) and wants (mostly a lot more money). So far (Prof. Bin Wu and Dr. George McNamara's management since 5/2017) our users needs are either (a) well satisfied by ourt confocal and other conventional microscopes, or (b) they go elsewhere for super-resolution and don't tell us.
| JHU iLabs Organizer | JHU / JHMI wide core resources |
iLabs Organizer ... we suggest you search for confocal https://johnshopkins.corefacilities.org/landing/42#/search |
|
| MicFac | East Baltimore - Physiology Bldg |
https://johnshopkins.corefacilities.org/service_center/show_external/3804 lots of equipment and staff. * Many confocal Microscopes ... mid-2019 prices: $29/hr, see https://microscopy.jhmi.edu/Services/fees.html * Four super-resolution microscopes. ... OMX, Nikon TIRF/dSTORM Super-Res, Lattice Light Sheet ... see URL above for rates. |
|
| MPI Core (Neurosci P30) | East Baltimore - PCTB |
http://neuroscience.jhu.edu/resources/3 multiphoton and confocal microscopy: Multiphoton Imaging Core Advanced Imaging and Physiology Resources For Common Use NINDS Institutional Center Core Grant To Support Neuroscience Research: Multiphoton Imaging (MPI) Core This is part of the Department of Neurosciences. their core's web site http://neuroscience.jhu.edu/resources/1 includes links to many JHU cores and mentions their department has a machine shop. |
|
|
Microscopy and Imaging Core Facility (MICF) |
East Baltimore - Robert H. and Clarice Smith Building Wilmer Eye Institute |
https://www.hopkinsmedicine.org/wilmer/research/micf Zeiss LSM880 AiryScan https://www.hopkinsmedicine.org/wilmer/research/core-centers/micf/equipment/zeiss-LSM880.html
|
|
| ICE | East Baltimore - Miller Research Bldg |
Institute for Cellular Engineering (ICE). Contact: Stewart Neifert. LSM880 with AiryScan and FastAiryScan, and LSM710 https://www.hopkinsmedicine.org/institute_cell_engineering/index.html https://www.hopkinsmedicine.org/institute_cell_engineering/about * G.I. Center members may be eligible for G.I. P30 Center financial contribution to help pay for your use of the ICE LSM880 (i.e. for AiryScan and FastAiryScan capabilities), please contact Prof. Donowitz or Dr. McNamara if interested. |
|
| BME | BME |
BME https://www.bme.jhu.edu/research/facilities-resources/ The department has a Nikon epifluorescence microscope equipped with a CCD camera for digital imaging. This unit is a core facility, available to departmental members at large. In addition, the department has an Olympus Fluoview 300, laser-scanning confocal microscope that is shared among several departmental laboratories that competed successfully for an NIH shared instrumentation grant. The confocal microscope is equipped for simultaneous electrophysiological and patch-clamp recording, and has custom lasers permitting CFP/YFP FRET imaging. |
|
| MRB |
MRB Molecular Imaging Service Center and Cancer Functional Imaging Core (MRB - miller Research Bldg) https://johnshopkins.corefacilities.org/service_center/3787/?tab=equipment (iLab Organizer) Multiphoton Intravital Microscope
|
||
| Cell Imaging Core Facility | The Sidney Kimmel Comprehensive Cancer Center |
https://www.hopkinsmedicine.org/kimmel_cancer_center/research_clinical_trials/research/shared_resources/cell_imaging.html https://johnshopkins.corefacilities.org/service_center/3000/?tab=equipment (iLab Organizer)
|
|
| Homewood - IIC |
http://pages.jh.edu/~iic/resources/instrumentation.html
|
||
| Carnegie embryology | Carnegie Institute for Science - Department of Embryology (next to JHU Homewood campus) |
https://emb.carnegiescience.edu One Leica STED nanoscope and lots more confocals. |
|
| HI^2 | BME & more |
Hopkins Imaging Initiative (BME centric, radiology, image processing, some microscopy) http://imaging.jhu.edu Welcome to the Hopkins Imaging Initiative (HI^2), a collaborative resource for all of the imaging research at Johns Hopkins University. HI^2 consists of Hopkins researchers who focus on image acquisition, analysis, and application. Some of our current goals: Connect researchers who develop and utilize imaging technologies
|
|
|
More cores (for various applications) and links https://www.hopkinsmedicine.org/research/resources/offices-policies/ora/handbook/corefacilities.html GCRC "web notes" http://www.hopkinsmedicine.org/webnotes/core_facilities (links to monthly calendar)
|
|||
| Other Baltimore Area Academic Confocal Core's | |||
| UMd SOM | UMd SOM | http://www.medschool.umaryland.edu/cibr/confocal | |
| NIH NIA IRP CIF Baltimore Bayview | NIH NIA IRP CIP Baltimore |
https://www.nia.nih.gov/research/labs/irp-core-confocal-microscopy-faciltiy IRP Core: Confocal Microscopy Faciltiy The Confocal Imaging Facility (CIF) provides state-of-the-art equipment, training, and image processing capabilities to assist all researchers of the NIA IRP in experiments involving light and confocal microscopy. The following major equipment is available: Zeiss LSM 880 confocal with Airyscan, 355 nm UV laser and including full live cell capabilities
|
|
|
NIH NIDA Baltimore Bayview |
NIH NIDA Baltimore Bayview |
https://irp.drugabuse.gov/organization/core-facilities/histology/equipment/ NIDA = National Institute on Drug Abuse Histology Core – Equipment and Services Olympus MVX10 - macroview zoom microscope Olympus VS120 - Virtual Slide Scanner TissueCyte 1000 - two-photon serial tomography scope LaVision Biotech Ultramicroscope II - light sheet fluorescence microscope. |





