q Andor Revolution X1 Spinning Disk confocal microscope - Legacy
Olympus Imaging Suite
Location: S910A
Andor Revolution X1 Spinning Disk confocal inverted microscope - Legacy instrument
Pease note: this microscope has been moved to Prof. Brian O'Rourke's lab. Please contact Prof. O'Rourke about its use.
Calendar: we initiatied iLab calendars for our other instruments (20191104Mon). The Andor is Legacy, so for now no calendar. If we start using it routinely for MetaMorph offline image analysis we will activate an iLab calendar for MetaMorph. Unlikely we will UN-legacy the Andor.
20190806Tue: the Andor X1 is now a Legacy instrument - still available for fully trained users, not actively being marketed to new users, not under any service contract: if a laser fails, or any other component fails, we plan to NOT look for funds to replace it/them (the P.I. of the one lab with an occasional user is welcome to provide money).. "Legacy" is an alternative to "full retirement"", which we will continue to consider. We do not currently need the space, so "legacy" is a useful status. The Andor X1 is controlled by MetaMorph, so at times when we saturate our other MetaMorph licenses, we can put users on this MetaMorph if needed (MetaMorph Premier license has more capabilities than our MetaMorph Basic offline licenses, but in practice, few analysis experiments need Premier's capabilities) . Andor X1 experiment users have priority over image analysis sessions ... in practice, this is being handled informally in that Andor X1 experiment users can make reservations as far in advance as they want (subject to our NOT promising the lasers etc will work), whereas MetaMorph image analysis reservations should not be made more than 3 work days in advance.
***
|
** Room lights policy (20190806Tue) whoever arrives first each day gets to control the main room light power switch at the front entrance. Thisis per our long standing policy: "Ross S910 overhead lights are controlled on "first come, first served" basis. That is, the first user to sign in and start their imaging session has control over all the overhead lights, and later users should abide by the settings of the first user." ==>Our thanks to Department of Medicine for funding and organizing the blackout curtains and track lights. |
|
News 20190709 (July 7, 2019): now uses Google Calendar for reservations (not this site's scheduler). The link is: ==>Make sure all your sessions are set to Public visibility. ==> Ross FISC calendars will move to iLab in October 2019. In the mean time, use Google Calendar (not this web site's calendar). |
Andor Revolution X1 Spinning Disk confocal inverted microscope.
* Andor 4 laser stack (405, 488, 561, 640 nm).
* Olympus IX81 inverted microscope
* Yokogawa CSU-X1 spinning disk scanhead CSUX1-M1N ... M1 = basic model (no widefield bypass), N = normal standard speed (5000rpm).
* MetaMorph acquisition.
* Andor TuCam image splitter, onto 2 cameras (i.e. green and red).
* Two Andor iXon 888 EMCCD cameras. Note: user is responsible for optimizing the EM gain settings (will typically NOT be identical).
TuCam device infromation
http://www.andor.com/scientific-cameras/multi-wavelength-imaging/tucam
TuCam cubes ... easiest way to see which set is in the TuCam is to find the cases for the cubes, whichever is empty is in the TuCam.
|
"1E" ... "Red / NIR" ... Cy3/Cy5, AF568/AF647 dichroic T605 LPXR Camera 1 emission filter: 575/50 ... 550-600 nm Camera 2 emission filter: 675/50M ... 650-700 nm |
|
"2B" ... "FITC/TRITC" ... "GFP / RFP" dichroic FF580-FDi01 x36 Camera 1 emission filter: 525/50 ... 500-550 nm Camera 2 emission filter: 617/73 ... 580-635 nm"3C" |
|
"3C" CFP/YFP dichroic FF509-FDi01 x36 Camera 1 emission filter: 475/28 ... 461-489 nm Camera 2 emission filter: 550/49 ... 525-575 nm Please note: 405 nm is not optimal for exciting "CFPs" and 488 nm is not optimal for exciting "YFPs". |
|
April 27, 2018 idea: Potential future filter set we would like your opinion of: Quad filters, to enable two sequential scans -> four fluorophores. this would be similar to how many point scanning confocal microscopes achieve "low bleedthrough 4plex". Laser line pairs: 405 and 561 ("blue and red") and 488 and 640 ("green and NIR") Microscope: Quad cube, i.e. DAPI/Green/Red/NIR Sutter emission filter wheel: pair of dual emission filters, Filter "A": blue, red. Filter "B": geen, NIR.. TuCam dichroic: "blue and red" toward camera 1, "green and NIR" toward camera 2. Emission filter for camera 1: dual "blue and red". Emission filter for camera 2: dual "green and NIR". Fluorescence onto cameras depends on laser lines and emission wheel. |
|
April 27, 2018 idea #2: confocal fluorescence anisotropy imaging microscopy (FAIM). Andor offers a polarizing beamsplitter & clean up filters for tghe TuCam, not clear whether the lasers are linearly polarized, nor if the whole light path is 'clean' with respect to POL. Equally or bigger question: do users really need this capability? Dix and Verkman 1990 publkished a nice paper ( https://www.ncbi.nlm.nih.gov/pubmed/2317548 ) measuring cytoplasmic viscosity by FAIM, and recently Ralph Weissleder has published several MP-FAIM articles, reviewsnd protocol papers. See: https://www.ncbi.nlm.nih.gov/pubmed/29410158 Fluorescence anisotropy imaging in drug discovery. Vinegoni C, Feruglio PF, Gryczynski I, Mazitschek R, Weissleder R. Adv Drug Deliv Rev. 2018 Feb 2. pii: S0169-409X(18)30027-9. doi: 10.1016/j.addr.2018.01.019. |
A simple way to get light to the TuCam is to set Sutter filter wheel "A" to "Quad" (the empty position also works), and sutter "A" shutter to open.
Key operating point: there is a shutter button on the Yokogawa spinning disk unit. I have not seen a Metamorph illumination control for it (yet), so user's responsibility to open the shutter by pushing a button on the Yokogawa X1 scanhead (the MetaMorph Acquire - Special tab has a 'camera shutter - always open' option that may or not do what you need).
tip: as a startinng point for optimizing the cameras (getting the settings optimized is user's responsibility), MetaMorph - Acquire - special tab screen shot:
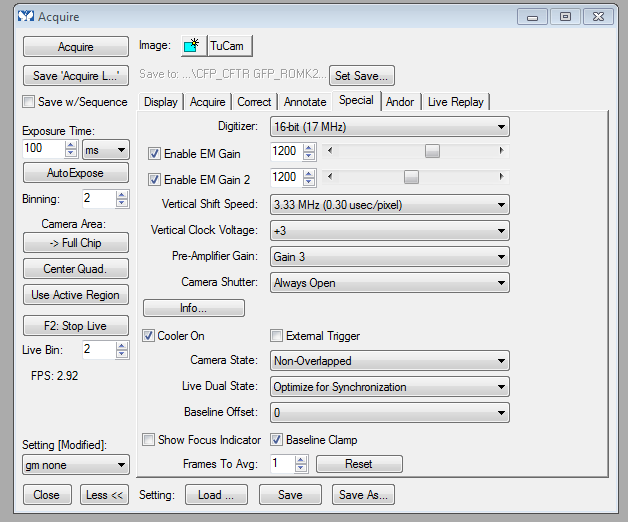
You want more dialogs? Sure:
Reserve Equipment


