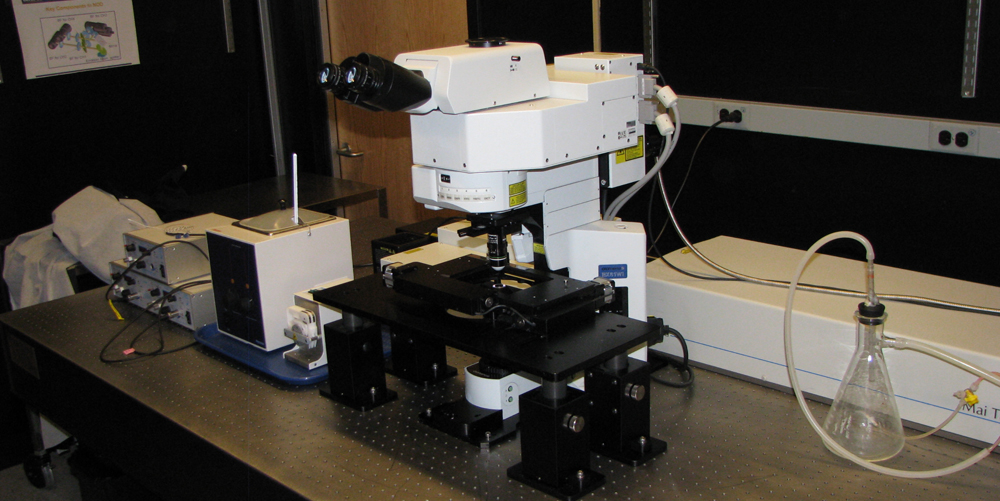
Olympus FV1000MP Multiphoton Excitation Fluorescence Microscope (MPEF) use iLab
Deep Tissue Scan Capabilities
Location: S972
Olympus FV1000MP Multiphoton Excitation Fluorescence Microscope (MPEF) ... also capable of SHG (ex. 840 nm --> 420 nm)
***
20191104M (November 4, 2019): we are now on JHU iLab for scheduling.
Ross Imaging Center
If a user needs their PI or administrator to set up account numbers (IO#'s in JHU accounting jargon), see
https://help.ilab.agilent.com/36900-managing-your-group/279959-membership-requests-fund-numbers
***
News 20170901:
Our FV1000MP does not currently have this capability (hint: a modest financial contribution from you might be able to add it; or please help us purchase a modern MPEF microscope with MP-FAIM capability, such as Olympus FV-MP) -- Ralph Weissleder et al published a terrific article on using multiphoton excitation fluorescence anisotropy (MP-FAIM) to image and quantify fluorescent conjugated drug binding specifically to the target protein ("receptor"). In this paper they used PARPi-BODIPY, with the unlabeled PARPi as a competitive inhibitor to block binding (and lots of other controls). They have separately published BRAF(V600E)i-BODIPY (Mikula et al 2017) and other fluorescent drug ligands.
Measurement of drug-target engagement in live cells by two-photon fluorescence anisotropy imaging.
Vinegoni C, Fumene Feruglio P, Brand C, Lee S, Nibbs AE, Stapleton S, Shah S, Gryczynski I, Reiner T, Mazitschek R, Weissleder R.
Nat Protoc. 2017 Jul;12(7):1472-1497. doi: 10.1038/nprot.2017.043.
PMID: 28686582
http://www.nature.com/nprot/journal/v12/n7/full/nprot.2017.043.html
***
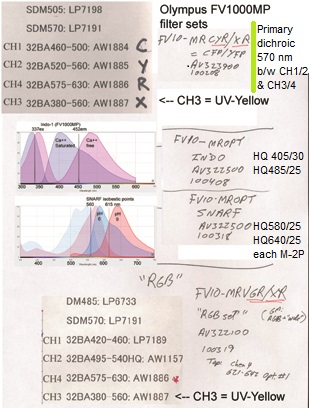
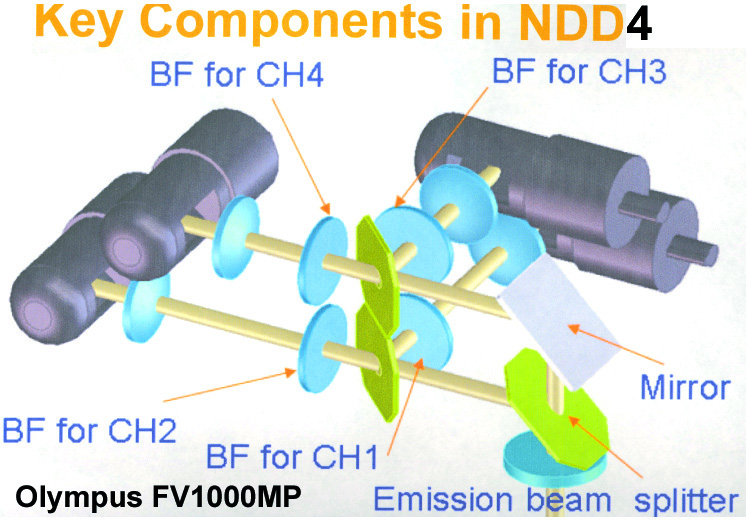
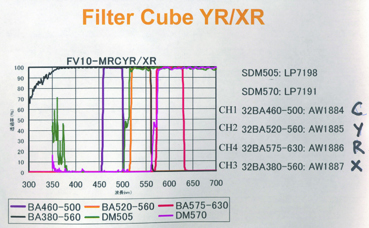
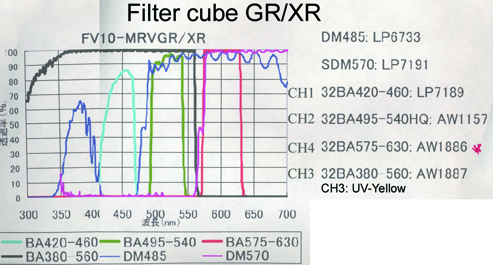
20190626Wed: here are our four filter cubes ... each is "double decker". In principle, we could buy additional empty cubes, plus appropriate beamsplitters and emission filters, for more fluorophores (new filters would likely have better performance because not 10 years old). In practice, no one has needed new filter sets.
Note; the microscope has a 570 nm dichroic (that does not explain why CH3 is typically >570 nm when all 4 PMTs are in use) to split the light between the lower deck (CH1& CH2) and upper deck (CH3 and CH4).
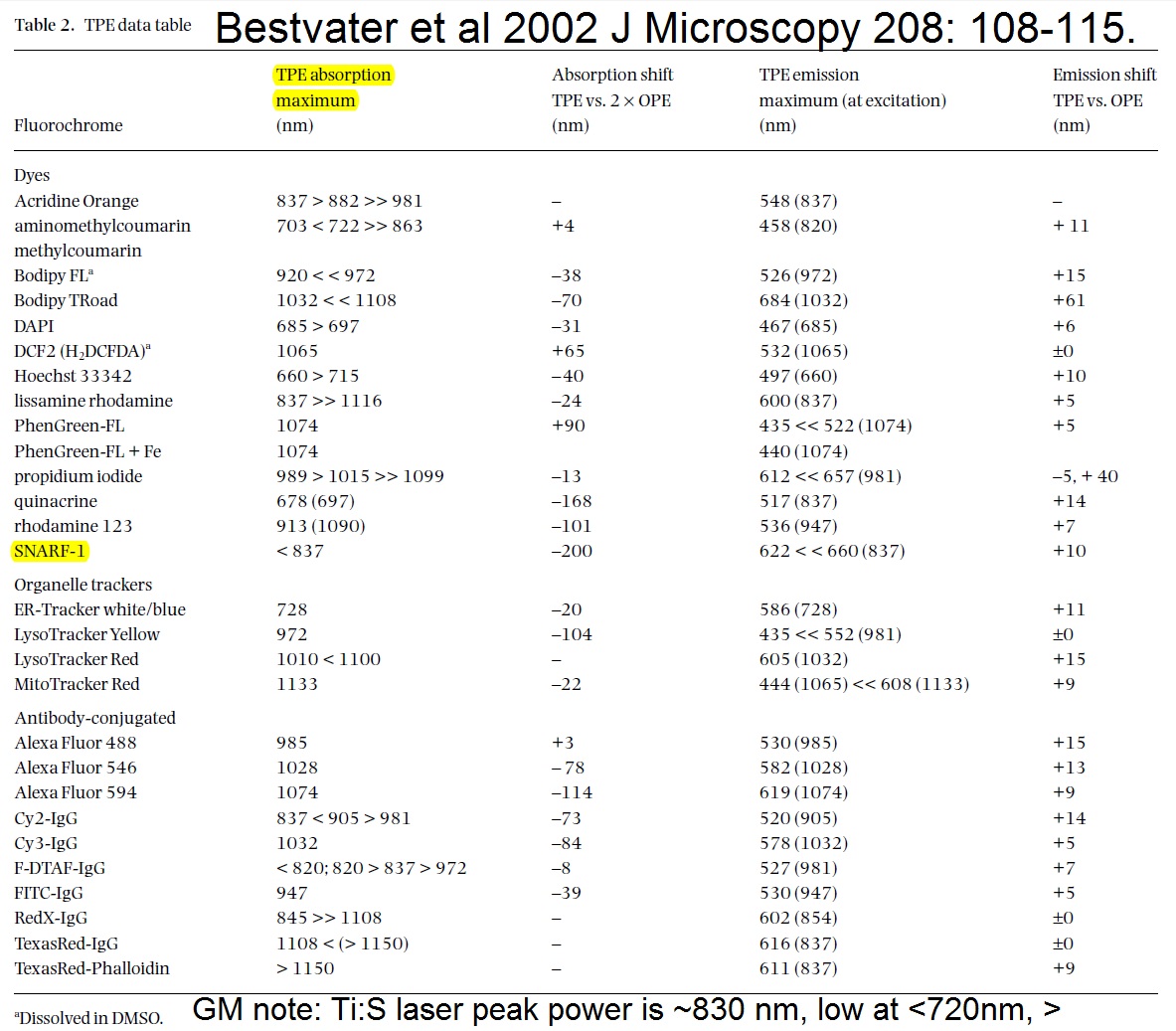
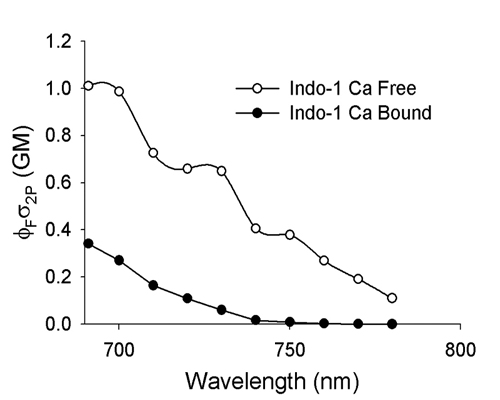
20190729Mon: info on SNARF and Indo-1 emission filters (each is "M-2P") ... the double decker cube dichroics are inside so no data on them ... typically these cubes are operated with the 570 nm "primary dichroic" OUT so all light goes to the upper deck:
Indo-1
HQ405/30 ... 393-417 nm
HQ485/25 ... 473-497 nm
SNARF
HQ580/25 ... 568-592 nm
HQ640/25 ... 628-652 nm
todays thought: perhaps could merge the INDO and SNARF into a single double decker cube (each now uses upper CH3 & CH4 with 570 nm primary dichroic out). ... then could purchase appropriate dichroics and emission filters for "modern fluorophores", especially Nathan Shaner's (201906 bioRxiv) AausFP1, 5x brighter than EGFP (and has a yellow version, should be similarly bright) plus Nathan's new "RFP that should make mCherry obsolete" and maybe mTurquoise2.See Talley Lambert's FPbase for info on fluorescent proteins, https://www.fpbase.org/chart/


|
|
|
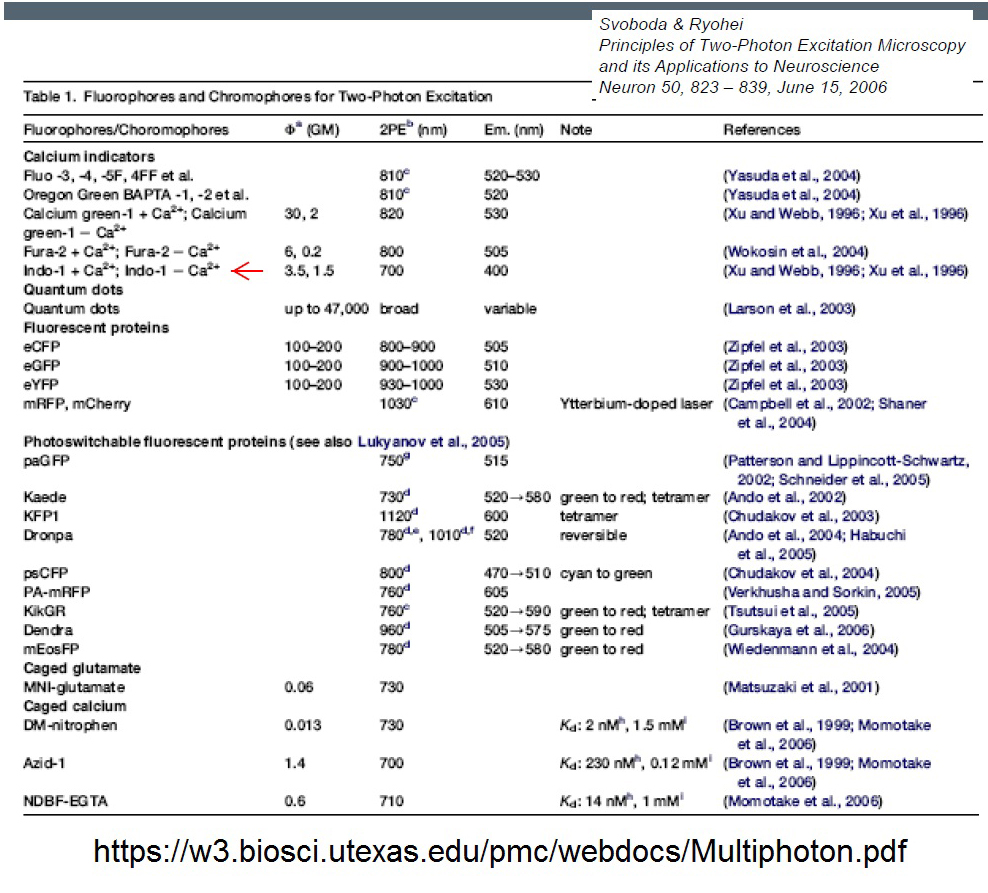
https://w3.biosci.utexas.edu/pmc/webdocs/Multiphoton.pdf
|
DrBio - Zipfel lab (Cornell University, co-invented MPEF with Prof. Watt Webb [and another colleague) is the classic source of 2-photon excitation spectra.https://zipfellab.bme.cornell.edu/cross_sections.html
|
Reserve Equipment