Multiplex
20210512W (start date - occasional edits) note: Multiplex Microscopy - also known as Spatial Biology
Some alternative terms: fluorescence image cytometry, spatialomics, spatial genomics (usually single molecule RNA, can be DNA), spatial proteomics.
Goal: maximize the amount of useful information, in as few stain-image-destain cycles as possible (ideally N=1), from each cell or 2D tissue section (most common(, or 3D tissue or organoid or whole-mount (organ, embryo, larva, adult animal [or plant]). This page mentions a few publications (by others) I think are useful (ex: Ce3D and IBEX from ron Germain's lab).
I have a long standing interest in multiplex -- aka multi-Probe -- fluorescence microscopy, having started "MPMicro" for a lecture at the Nikon AQLM course at MBL in 5/1995 (26 years ago on starting this page 5/2021). MPMicro is available online at https://works.bepress.com/gmcnamara/2 (please do not hit the print button).
McNamara et al CPHG Unit - open access (I negotiated open access for this Unit in exchange for me doing the update - in 2022 I was invited to do another update and declined, so future unit will have a new first author and possibly removal of myself and/or other authors):
web page https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cphg.42
PDF https://currentprotocols.onlinelibrary.wiley.com/doi/epdf/10.1002/cphg.42
See tables with many fluorophores, including "Brightness" calculation. Please note: a few have errors (NIR4, STAR Red) - if you see errors, please email me.
Older version of the tables (also a few errors) at https://www.geomcnamara.com/data
Below is a summary I posted on linkedin and add some more text here -- the linkedin page has some graphics of spectra (we have hit a limit on our web hosting site * budget wrt graphics here), see also https://searchlight.semrock.com and other spectra search web sites, or if you prefer Microsoft Excel data, my (2006) PubSpectra data set is at https://works.bepress.com/gmcnamara/9
|
GM Suggestion = Hypothesis: Could Multiplex different brands using the same excitation and emission -- especially if you have the right microscope, such as a spectral fluorescence lifetime imager (you could buy the image core a fully loaded Leica STELLARIS 8 with 5 HyD S SiPMs inside, and 4 more on X1 port, for us to figure out - other brands&models possible), and right software. Easiest if the different markers are in different locations, such as different cells. For example, may be able to multiplex ALL the BD Brilliant Ultraviolets (BUV's) with Bio-Rad's StarBright UltraViolets (SBUVs) and add in fluorescent quantum dots (QDots). Repear with Brilliant Biolets, SBVs, etc. |
https://www.linkedin.com/pulse/superbright-tandems-outed-thermofisher-now-has-plus-series-mcnamara/
Brilliant Ultraviolets, Violets, Blues, Yellow-Green(s) ... BD Biosciences (some BVs available BioLegend, Jackson Immunores). ... See below for possible Alexa Fluor acceptors of BV's.
Super Bright ... ThermoFisher
Super Nova ... Beckman Coulter
StarBright ... Bio-Rad ... see below ... StarBright Ultraviolet (340nm excitation max, should excite at 368nm wrt FISHscope], StarBright Violet, StarBright Blue ... 2023: Yellow, Red
Above are, or likely, "polymer dyes", based on Nobel Prize in Chemistry 2000, http://sirigen.com/sirigen_technology.html (Sirigen was acquired by BD Biosciences - more on this below).
How similar are BV's and SuperNova's? ... patent case(s) may provide answer https://www.maxval.com/blog/becton-dickinson-filed-a-case-against-beckman-coulter-over-alleged-patent-infringement/ ... not that this will affect customers too much, though some patent lawyers and law firm(s) likely to be the big financial winners.
Brilliants were invented by Sirigen (acquired by BD Biosciences), which was cofounded by 2000 Chemistry Nobel laureates to commercialize their discovery of electron carrying polymers.
--
some recent fluorophore families based on other technologies:
Nova Fluor (NovaFluor) ... Phitonex, acquired by ThermoFisher ... based on what I refer to as DNA origami (Duke U. spin out). More on NovaFluor's below (19plex 202111 - 17plex spectra, plus 2 deliberately dimmer variants).
20230207 email from ThermoFisher Invitrogen: 3 More NovaFluors: Yellow 755 Red 725 Red 755. See also web page below
CPN ... StreamBio UK ... conjugated polymer nanoparticles https://www.streambio.co.uk (may need to wait for LinkBright product line to get into Sigma-Aldrich web site to avoid crazy shipping charges from U.K.). More on CPNs below.
StabiLux Biosciences - https://stabiluxbiosciences.com/ - NovoLux ... lacks details on the technology.
==> I am happy to add other academics and vendors weblinks here ... please contact me on linkedin and/or my JHMI email (see "About Us"). Note: I do not have any commercial interest in any company involved in fluorescence.
***
more options ...
Alphathera (2/2023) announced oYo-Link Horseradish Peroxidase
| https://alphathera.com/enzyme-conjugation/p/horseradish-peroxidase-hrp oYo-Link Horseradish Peroxidase oYo-Link HRP enables the rapid production of highly uniform primary antibody-HRP conjugates in under 2 hours, with only 30 seconds hands-on time. Site-directed conjugation of 1-2 HRP labels to the heavy chain of compatible primary antibodies ensures consistent conjugates that do not require optimization. Simply mix the oYo-Link® HRP with your antibody and then illuminate using our LED Photocrosslinking device. |
|
How you might use: oYo-Link is a covalent (UV light - they sell a nice source) crosslinking method to conjugatwe HRP-(propreitary variant of ProteinA/G) onto your unlabeled antibody (FYI: occludes FcRn binding; does NOT affect FcR binding) ... in this case HRP-oYo-Antibody. So you could 1. generate in your lab HRP-oYo-Link to EACH of your antibodies. 2. buy Alexa Fluor tyramide signal amplification DYE-TYRAMIDE conjugates and H2O2. 3. Apply one HRP-oYoLink-antibody, then fluorophore-tyramide + H2O2 ... hundreds to thousands of dye-tyramide covalently bond to tyrosines (and His, Trp) on your specimens proteins. 4. "Kill" HRP ... I recommend BioCare Medical's PeroxAbolish (Akoya and other companies like to cook the specimens - probably overcook and strip many of the tyramides, hence I like PeroxAbolish). 4. Next round with a different fluorophore-tyramide. oYoLink is going to attach ONE (or maybe a couple?) HRP per antibody, so not as much enzyme as (HRP-polymer)-Ab that pathology labs typically use for DAB IHC. You could mix and match as needed. |
Alphathera (4/2022) announced new product(s) for their oYo-Link lableing system with the ability to install any of 10 peptide epitope tags onto IgG antibody heavy chain, see
https://alphathera.com/news/alphathera-launches-the-oyo-link-epitope-tag-for-enhanced-multiplexing-capability
https://alphathera.com/epitope-tag/p/epitope-tag
Specifications table (molecular weight in parentheses, kD):
His12 Tag (8.7)
DYKDDDDK Tag (12.2)
V5 Tag (13.4)
S Tag (14.4)
VSVg Tag (13.2)
NWS Tag (12.7)
S1 Tag (12.5)
AU1 Tag (11.7)
AU5 Tag (11.6)
HSV1 Tag (13.1)
See https://alphathera.com/all-products for all their products - all make covalent bond, typically ProteinG-IgG heavy chain (UV activated site specific crosslink).
**
GM 20211130 -
Philosophies to Dye for ...
- In theory, the product of extinction coefficient * quantum yield, should enable rank ordering of fluorophores. I usually use Ec*QY / 1000 = “Brightness” (B), which for fluorescein (pH 9) is 90,000 * 0.9 / 1000 = 81.
- In practice, “Brightness” calculation is free dye in solution, not when bound to antibody or oligonucleotide.
- Brightness of antibody-dye conjugates, whether commercial or made in house, is also subject to issues of degree of Labeling (DoL), with antibody with high DoL usually self-quenching (low fluorescence).
- Commercial special case: a company nicknamed “Santa Crap” should simply be avoided.
- Performance of dyes – especially in context of multiplexing (due to cross talk) – in part depends on what the microscope is able to excite well and collect emission from well. FISHscope is more versatile than most microscopes (but is not perfect).
- McNamara 2017 CPHG has table of fluorophores with Brightness calculation – starting on page 50. Online version at https://www.geomcnamara.com/fluorophore-table (used to be able to sort by any table column – that no longer works).
I came across Alexa Fluor 610-X as being the brightest small molecule fluorescent dye of ~40 tested by Maillard et al 2020 Universal quenching of common fluorescent probes by water and alcohols (yes, you read that correctly: water quenches fluorophores, D2O does not). Chem Sci 12: 1352. https://pubs.rsc.org/en/content/articlelanding/2021/sc/d0sc05431c (also discussed below).
The Brilliants, SuperBrights, etc, summarized above, andfurther discussed below, are probably less susceptible to the H2O quenching phenomenon.
***
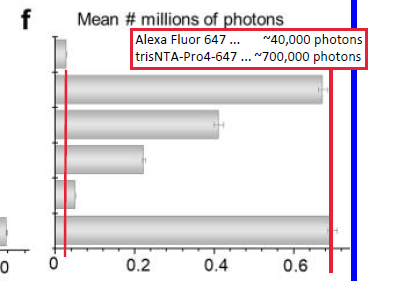
20220210U: Brief note - many ways to improve fluorophore behavior. One, that fits nicely with recombinant proteins (genes) with 6His tag added, is to use Ni+ : NTA, at optimal distance, to quench fluorophore triplet state (dark state) while permittign singlet excited state to emit photon (fluorescence). Example: Alexa Fluor 647 without protection 40,000 photons, trisNTA-AF647 ~700,000 photons (almost 20x more photons - see fig2F below). Nice paper:
Glembockyte V, ... Costa, Tampe 2018 Tris-N-Nitrilotriacetic Acid Fluorophore as a Self-Healing Dye for Single-Molecule Fluorescence Imaging. J Am Chem Soc 140: 11006−11012. DOI: 10.1021/jacs.8b04681. NTA-linker-fluorophore
20211029 update: "Best and Brightest" note
I am big on BV421 and other modern fluorophores because some of these greatly improve on fluorescence Brightness, where Brightness = E.c. * QY / 1000 (Extinction coefficient, Quantum yield), ex.BV421 B=1500, fluorescein = 81. this is like getting paid $1500/hr vs $81/hr (to complete the analogy, if you get paid $100/hr as a round number and work 2,080 hours per year, this would be $208,000 annual salary, $81*2250 = $168,480 vs $1500*2250 = $3,120,000 ..which would you prefer?). Yet, many biologists still use fluorescent (ex. "FITC conjugated" secondary antibody, or Alexa Fluor 488 Brightness = 67 (pH insensitive and more photostable than fluorescein, B=67 from https://www.geomcnamara.com/fluorophore-table ).
I came across Maillard et al 2020, who compared 42 fluorophores. I was amazeed to see that Alexa Fluor 610-X ("AF610X") a fluorophore I had never paid attention to, was 2.4x brighter than Alexa Fluor 594 (which spectrally, and maybe chemically, is similar to Texas Red, one of the first fluorophores the founders of Molecular Probes developed ... they eventually sold to Invitrogen, which was acquired by ThermoFisher). Again: would you like to get paid 2.4x more than you do now? ... FYI (I believe) ThermoFisher/Molecular Probes "custom conjugation" group can put AF610X on any antibody in their catalog, and sell to you at same price as "catalog" antibodies. I also note that some Dyomics ("DY") fluorophores had high Brightness values. I do not Brightness value is not everything: performance at various degrees of labeling (DoL) on antibody, photostability, lack of going into triplet excited state and/or other dark states (which are good for PALM/STORM precision localization microscopy), reactive oxygen species (often consequence of going into triplet states) play a role in success.
Maillard J et al 2021 Universal quenching of common fluorescent probes by water and alcohols. Chem. Sci. 12: 1352. https://pubs.rsc.org/en/content/articlelanding/2021/sc/d0sc05431c
Alexa Fluor 610-X highest Brightness B=121 in H2O (highest Brightness of the 42 dyes), over 2x that of Alexa Fluor 594, B=54 in H2O. ... Alexa Fluor 610-X in D2O is B=140,
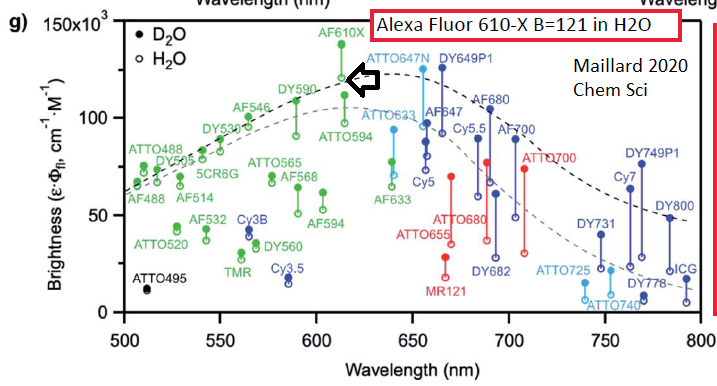
see also https://www.linkedin.com/pulse/maillard2020-chemsci-42-fluorophores-paper-values-george-mcnamara/
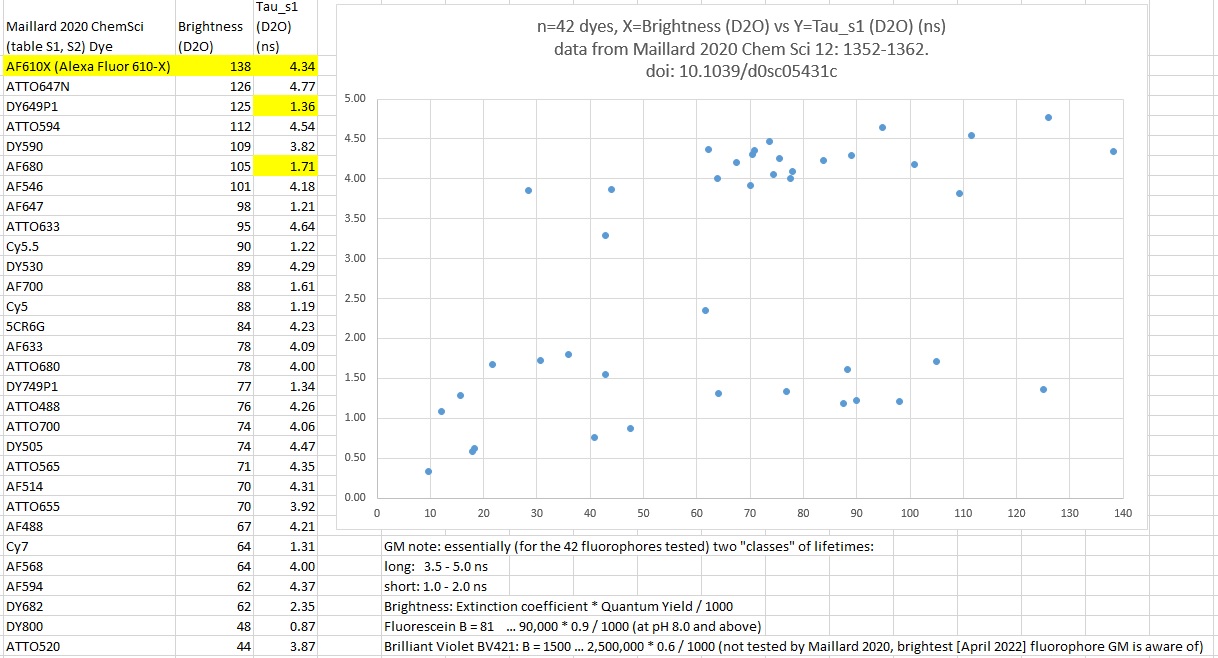
Another look at Maillard 2020 ChemSci data: two 'classes" of fluorescence lifetimes, long, 3.5-5.0 ns, and short, 1.0-2.0 ns. (all 42 fluorophores graphed, on brightest tabulated, since you should avoid "unbright" fluorophores - Excel sheet available from me). These are "in D2O" values -- the "in H2O" proportionally dimmer and shorter lifetimes (not shown, available in my Excel sheet based on data from their supplemental PDF). I note that X-axis of their figure panel "g" above and "my" Brightness vs Tau below are the same (Brigrness). ... Yes, the graph below does not have spectral info - so what? You cvan get the Excel file from me or figue it out for yourself. I suggest a kick-butt imaging system would be a Leica STELLARIS 8+++ (or how about ST9 !) ... STELLARIS FALCON with 8 HyD S (SiPMs) and one HyD R (NIR, for wavelengths range that "R" outperforms "S"), wgite light lase wit NKT Photonics UV-Extend (down to 350nm excitation), ALL the best ~4ns and best ~1ns fluorophores, plus the Brilliant Ultraviolets (BUV, ~350nm excitation hence UV-EXTEND), Brilliant Violets (~395 excitation, also UV-EXTEND), Brilliant Blues (only ~5), and best dyes below (tyramide signal amplification [TSA] or methyl-luminol (may be 10x better than tyramide) ... potentially 8plex or more chlorins (see NIRVana Sciences content on this page). --> say 40plex in one acquisition. I note Leica STELLARIS could be operated in "push-broom" ode, of line scan one axis, move stage in other axis, as big as specimen (ex. adult human brain slice or liver slice).
20220504W same data as above (plus some Abberior dyes) with new graph using emission wavelength maxima - then data table below.
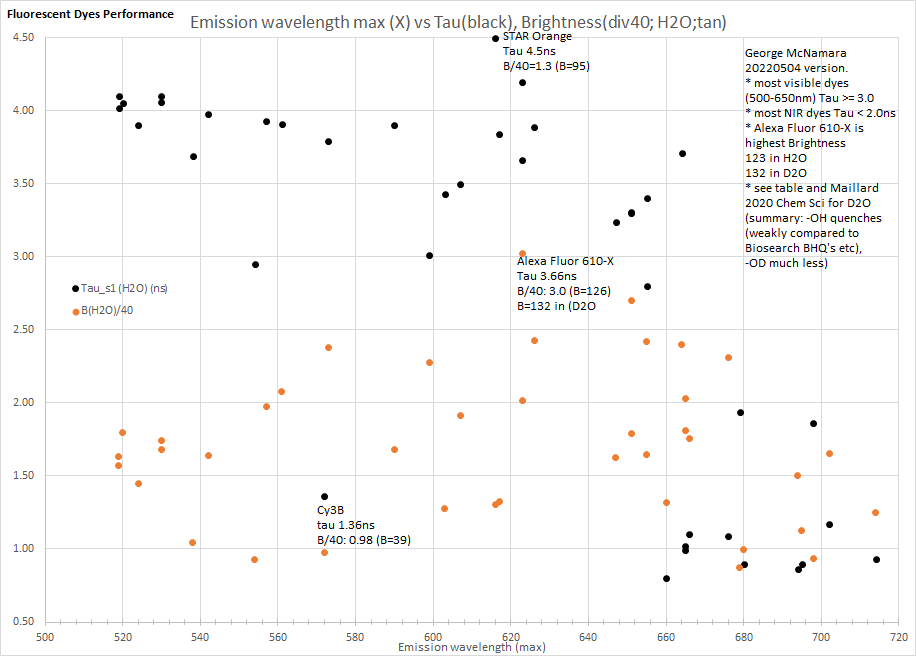
Maillard 2020 Dye wavelength em max (nm) Tau_s1 (H2O) (ns) B(H2O)/40 Brightness (H2O) [you can fix formatting by copying to Excel etc]
AF488 519 4.0 1.6 65
STAR GREEN 519 4.1 1.6 63
ATTO488 520 4.1 1.8 72
STAR 488 524 3.9 1.4 58
DY505 530 4.1 1.7 67
STAR 512 530 4.1 1.7 70
ATTO520 538 3.7 1.0 42
AF514 542 4.0 1.6 66
AF532 554 3.0 0.9 37
5CR6G 557 3.9 2.0 79
DY530 561 3.9 2.1 83
Cy3B 572 1.4 1.0 39
AF546 573 3.8 2.4 95
ATTO565 590 3.9 1.7 67
DY590 599 3.0 2.3 91
AF568 603 3.4 1.3 51
STAR 580 607 3.5 1.9 77
STAR ORANGE 616 4.5 1.3 52
AF594 617 3.8 1.3 53
AF610X 623 3.7 3.0 121 Alexa Fluor 610-X
STAR 600 623 4.2 2.0 81
ATTO594 626 3.9 2.4 97
AF633 647 3.2 1.6 65
STAR 635P 651 3.3 2.7 108
ATTO633 651 3.3 1.8 72
STAR 635 655 2.8 2.4 97
STAR RED (KK114) 655 3.4 1.7 66
FLUX 640 (Abberior) 660 0.8 1.3 53
ATTO647N 664 3.7 2.4 96
Cy5 665 1.0 1.8 73
AF647 665 1.0 2.0 81
FLUX 647 666 1.1 1.8 70
DY649P1 676 1.1 2.3 93
ATTO655 679 1.9 0.9 35
FLUX 660 (Abberior) 680 0.9 1.0 40
Cy5.5 694 0.9 1.5 60
FLUX 680 (Abberior) 695 0.9 1.1 45
ATTO680 698 1.9 0.9 38
AF680 702 1.2 1.7 66
AF700 714 0.9 1.2 50
**
Some deuterated pricing (April 20, 2022)
https://www.sigmaaldrich.com/US/en/product/aldrich/151882
151882-10G ... $28.50 ... 10 grams is ~10 mL
151882-100G ... $152.00
151882-1KG ... $1,320.00
for comparison (more viscous, a'la VectaShield)
https://www.sigmaaldrich.com/US/en/product/aldrich/447498
Glycerol-d8 (fully deuterated)
447498-1G ... $159.00
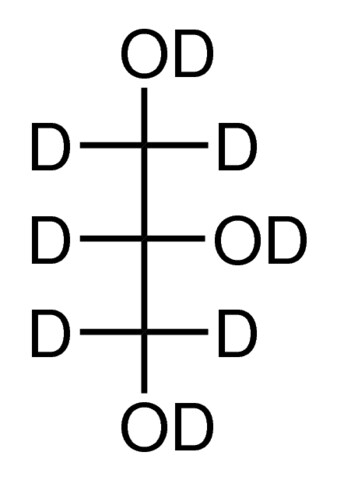
I suspect (but I'm no chemist) based on my understanding of Maillard et al 2020 ChemSci that only the OH's need to be replaced with OD's for deuterated glycerol to be effective -- that is to minimize quenching of fluorophores. Glycerol is traditionally used in fluorescence microscopy for both refractive index increase compared to water and to increase viscosity. The latter limits diffusion of O2, so usually best practice to avoid vortexing glycerol solutions (plus, vortexing, shaking, stirring would get air bubbles into the media, which would mess up image quality).
***
ThermoFisher has a brief table of some of the basic parameters of some of their fluorophores - but not AF610-X (Maillard 2020 supplemental pdf has fluorescent lifetimes in H2O and D2O for all 42 tested -- I generated an Excel table from their data and calculated Brightness values - on my linkedin page).
|
20220118U: please see publication and especially supplemental table for mre details on this. Fluorophore (fluorescent dye) free in solution vs conjugates can have different fluorescence lifetimes ("Tau") and quantum yields (Q.Y.) -- and different linker lengths and "local environment" (such as very nearby guanine on oligonucleotide, or tryptophan adjacent to dye on proteins) - as nicely shown in the example below. GM notes that higher Tau and QY usually "go together". Niehorster ... Sauer 2016 Multi-target spectrally resolved fluorescence lifetime imaging microscopy. Nat Meth 13: 257-262. https://www.nature.com/articles/nmeth.3740 doi:10.1038/nmeth.3740 GM note: ideally would use site specific conjugation, such as C-terminal amino acid of nanobody (12 kDa VHH only), or single site on a monoclonal antibody (mAb ~155 kDa, could be "cut down" with enzyme to ~50 kDa Fab), instead of "random lysines" on each IgG antibody molecule of a population of polyclonal antibodies (~155 kDa, lots of different amino acid sequences in population, and random lysines, such as degree of labeling DoL ~3 out of ~20 surface lysines on average). ???Missed opportunity by these authors???: FRET or its quenching variant (QRET) can be used to "tune" donor lifetime (moreFRET results in on average shorter donor lifetime) AND acceptor lifetime has interesting behavior (over statistics of many events counted) in that there is a delayed rise in acceptor lifetime when FRETting. That is FLIM-FRET of donor-acceptor, especially with defined site labeling (ex. COOH of nanobody) and linker length between conjugate and dye, and of linker (spacer) between donor and acceptor. Potential additional degree of freedom: conjugate-linker-donor-spacer-acceptor and conjugate-linker-acceptor-spacer-donor may behave differently even if linker and spacer are the same in each. |
As noted above (Alexa Fluor 488 B=67) Brightness of many fluorophores are tabulated at https://www.geomcnamara.com/fluorophore-table and in our 2017 CPHG (open access)
https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cphg.42
Back to Dyomics (several DY dyes had high Brightness in Maillard 2020) ... their products https://dyomics.com/en/products include several families of MetaStokes dyes. Many vendors do not include extinction coefficient (Ec) or quantum yield (QY) and/or "MegaStokes" aka "Long Stokes Shift" (LSS) dyes have odest or low quantum yields. If you want to use MegaStokes dyes to get the ability to excite more fluorophores with the same LED or laser line, either "just do it" (order the stuff and try it), or look at Brilliants, Nova Fluors, StreamBio CPNs and/or other similar modern fluorophores.
***
20220524Tue: Additional Long Stokes Shift dyes ("LSS", most are modestly longer Stokes shifts)
--> LSS facilitates multiplexing by using the same excitation wavelength for multiple fluorophores with different Stokes shifts [Stokes shift = emission maximum wavelength minus excitation maximum wavelength). This web page summarizes several other ways to get multiple fluorophores of different emission maxima -- and spectra -- from each excitation wavelength. These include (not limited to): Brilliants and similar fluorescent polymers (polymer with conventional flurophore attached), Stream.Bio CPNs, ThermoFisher Nova Fluors (acquired Phitonex, using DNA origami to position donor and acceptor fluorophores at precise distance and Kappa squared orientation for FRET).
--> Jiang et al compared azetidine rings ("square rings" from Luke Lavis lab) and other fluorophore modifications and (not surprisingly) found their new modification best.
Jiang G ... Yan L 2022 A synergistic strategy to develop photostable and bright dyes with long Stokes shift for nanoscopy.
Nat. Communs 13:2264 https://doi.org/10.1038/s41467-022-29547-3
Name λab(nm) λem(nm) Stokes shift/nm φ ε(M−1 cm−1) ε × φ/1000 Δ(ε × φ) [YL vs parent] Scaffold
YL578 578 634 56 0.74 89,000 66.4 2 Rhodamine
RhB 553 580 27 0.31 105,000 32.4
11 548a 612a 64 0.62a 51,000a 31.6 2.5
R-2 518 546 29 0.21 60,000 12.6
12 577a 623a 46 0.71a 83,300a 59.1 3.2
R-3 552 581 24 0.18 103,000 18.5
13 406b 513b 117 0.55b 32,400b 17.8 5.2
R-4 372b 470b 98 0.19b 18,000b 3.4
14 464b 556b 92 0.67b 26,200b 17.5 8.1 Coumarin
R-5 430b 484b 54 0.06b 32,200b 2.2
15 439b 575b 136 0.40b 25,100b 10.0 8.0 Boranil
R-6 401b 462b 61 0.04b 31,300b 1.3
aMeasured in PBS (25 mM, pH 7.4).
bMeasured in PBS (25 mM, pH 7.4) containing 20% EtOH. Δ(ɛ × φ) represents the ratio of the increased brightness.
***
Brilliants were developed by Sirigen (acquired by BD Biosciences for $55M) using technology (and patents) developed out of work that won the Nobel Prize in Chemistry in 2000, see
http://sirigen.com/sirigen_technology.html
http://sirigen.com/microscopic.html
see also BioLegend (acquired by PerkinElmer ... BioLegend licensed BV's from Sirigen, but do not have access to BUVs, BBs, BYGs) Brilliant Violets web pages
https://www.biolegend.com/en-us/brilliant-violet web pages [About, Technology] stating BV421 has extinction coefficient 2,500,000 M-1cm-1, quantum yield 0.65, so Brightness (Ec*QY/1000) ~1500 vs fluorescein B=81
20211011Mon - GM table of POSSIBLE BV421 donor --> Alexa Fluor acceptors (I had previously identified BV570 as BV421 -> AF546, more candidates today - 480 and 510 are different polymers than BV421 (have different spectra ... which comes from different chemistry).
20220119W found nice history of Sirigen and Brilliants, and alternative possible fluorophores (some of which do not make spectral sense to me - and no idea where their Stokes shift math comes from, gven 421 - 407 = 14)
L. Della Ciana, 2019 New Trends in Fluorescent Reporters in Biology in Springer Series of Fluorescence 19. Author affiliation: Cyanagen srl, Bologna, Italy (see for some products, https://www.cyanagen.com/search-for-applications/ ). (page 335) Table 5 Optical properties of Brilliant Violet polymer dyes
DY682 and other Dy dyes are from Dyomics, ex https://dyomics.com/en/products/far-red-excitation/dy-682 (unfortunately not in Semrock SpectraViewer)
| Brilliant Violet | Alexa Fluor acceptor - GM hypothesis | Della Ciana table 5 | ex | em | Stokes Shift (Della Ciana) |
| BV421 | base polymer (of all tandems below) | 407 | 421 | 106 | |
| BV480 | base polymer | - | - | ||
| BV510 | base polymer | 405 | 510 | 133 | |
| BV570 | Alexa Fluor 546 | Cy3 | 407 | 570 | 168 |
| BV605 | Alexa Fluor 568 | Cy3.5 | 407 | 605 | 175 |
| BV650 | Alexa Fluor 633 | Dy610 | 407 | 650 | 261 |
| BV711 | Alexa Fluor 700 | Dy682 | 407 | 711 | 304 |
| BV750 | - | 407 | 750 | ||
| BV785 | Alexa Fluor 750 | Dy752 | 407 | 786 | 379 |
Table note: I used https://searchlight.semrock.com to match fluorophorer spectra - not exact matches: the emission peaks are very close, some of the emission tails have "bumps", some do not.
If I am correct, one could use 405nm to excite ALL the Brilliant Violets, and "appropriate laser line(s)" to excite stand alone Alexa Fluor's as antibody conjugates (or tyramide signal amplification products), single molecule RNA FISH, DNA-PAINT, etc. Yes, the BV-->acceptor mighthave some signal on direct acceptor excitation, butcould be corrected by "spectral unmixing" (multichannel unmixing) vs the BV-acceptor.
If anyone finds better candidate(s) for ecceptors, please email me suggestions to update here.
I use Semrock Searchlight for spectral comparisons, https://searchlight.semrock.com/# (login required for full benefit of the spectral web site).
**
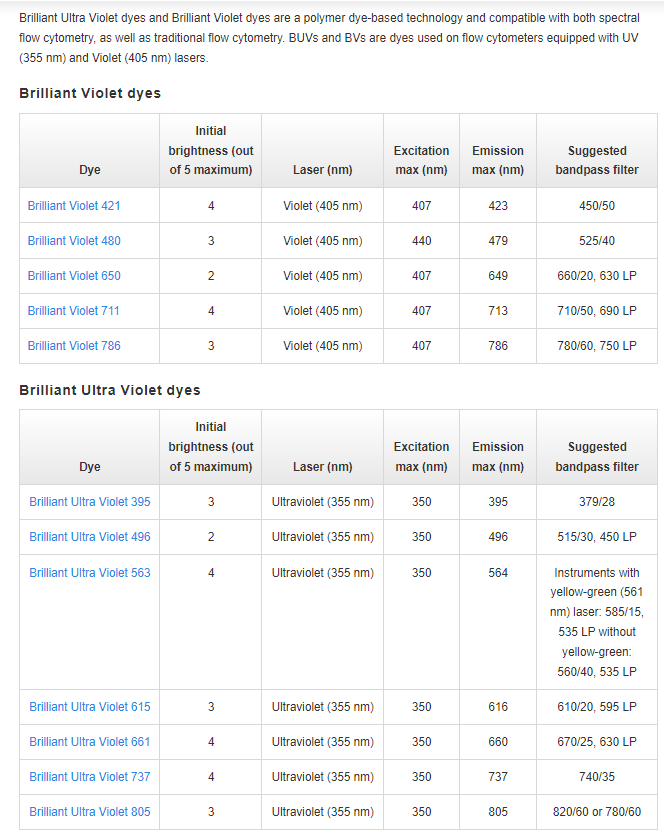
20230224F update: ThermoFisher/Invitrogen posted online a table of "Initial Brightness" etc of BV's and BUV's - somewhat ironic since they compete by ALSO selling SuperBrights (that do not get any love on that web page - is mentioned in the marketing email to the link). I note that a five point scale is not an especially useful way to present how bright (i.e. ideally photons/sec, under some standard illumination and detection settings) a fluorophore will be.
https://www.thermofisher.com/us/en/home/life-science/cell-analysis/flow-cytometry/brilliant-polymer-dyes.html
Table(s) in text below, then graphic (screen shot from ThermoFisher web page)
"B" = Initial Brightness out of 5 maximum
Polymer B Laser (nm) Ex max Em max Suggested bandpass filter
Brilliant Violet 421 (BV421) 4 Violet (405 nm) 407 423 450/50
Brilliant Violet 480 3 Violet (405 nm) 440 479 525/40
Brilliant Violet 650 2 Violet (405 nm) 407 649 660/20, 630 LP
Brilliant Violet 711 4 Violet (405 nm) 407 713 710/50, 690 LP
Brilliant Violet 786 3 Violet (405 nm) 407 786 780/60, 750 LP
Brilliant Ultra Violet dyes
Polymer B Laser (nm) Ex max Em max Suggested bandpass filter
Brilliant Ultra Violet 395 3 Ultraviolet (355 nm) 350 395 379/28
Brilliant Ultra Violet 496 2 Ultraviolet (355 nm) 350 496 515/30, 450 LP
Brilliant Ultra Violet 563 4 Ultraviolet (355 nm) 350 564 Instruments with yellow-green (561 nm) laser: 585/15, 535 LP without yellow-green: 560/40, 535 LP
Brilliant Ultra Violet 615 3 Ultraviolet (355 nm) 350 616 610/20, 595 LP
Brilliant Ultra Violet 661 4 Ultraviolet (355 nm) 350 660 670/25, 630 LP
Brilliant Ultra Violet 737 4 Ultraviolet (355 nm) 350 737 740/35
Brilliant Ultra Violet 805 3 Ultraviolet (355 nm) 350 805 820/60 or 780/60
**
BioLegend fluorophore families
|
BioLegend 20211012U (plus updates) https://www.biolegend.com/en-us/fluorophore-families https://www.biolegend.com/en-us/spark-dyes Spark Violet 423 ex 405nm ("423" added early 2022) KIRAVIA Blue 520 ex 488nm ("distinct class of multimers with distinct chemistry" ... 8 kDa) APC/Fire 750 ex 650nm (or 638nm, 640nm) BioLegend also offers Brilliant Violets (BV###) - see above-- licensed from Sirigen before Sirigen was acquired by BD Biosciences (BD also offers Brilliant Ultraviolets BUV###, Brilliant Blues BB###, Brilliant Yellow-Green BYG###).
|
***
11/2021 ThermoFisher NovaFluors ... ThermoFisher acquired Phitonex 12/2020.
Product info from https://www.thermofisher.com/us/en/home/life-science/cell-analysis/flow-cytometry/novafluor-dyes.html - see web page for excitation and emission maxima. I note there is potential to use fast FLIM (fast fluorescence lifetime imaging microscopy, examples: Leica STELLARIS 8, Becker&Hickl, PicoQuant, ISS) to distinguish the 610/30S from 610/70S and the 660/120S from 660/40S pairs -- that is, the brighter product (high #S) should have shorter donor lifetime, and dimmer product (lower #S) longer donor lifetime -- each shorter than the donor alone product NovaFluor Blue 510. .
1 NovaFluor Blue 510
2 NovaFluor Blue 530
3 NovaFluor Blue 555
4 NovaFluor Blue 585
NovaFluor Blue 610/30S (dimmer acceptor)
6 NovaFluor Blue 610/70S (brighter acceptor)
7 NovaFluor Blue 660/120S (dimmer acceptor)
NovaFluor Blue 660/40S (brighter acceptor)
9 NovaFluor Red 660
10 NovaFluor Red 685
11 NovaFluor Red 700
12 NovaFluor Red 710
13 NovaFluor Yellow 570
14 NovaFluor Yellow 590
15 NovaFluor Yellow 610
16 NovaFluor Yellow 660
17 NovaFluor Yellow 690
18 NovaFluor Yellow 700
19 NovaFluor Yellow 730
(for clarity, TM trademarks removed from NovaFluor names).
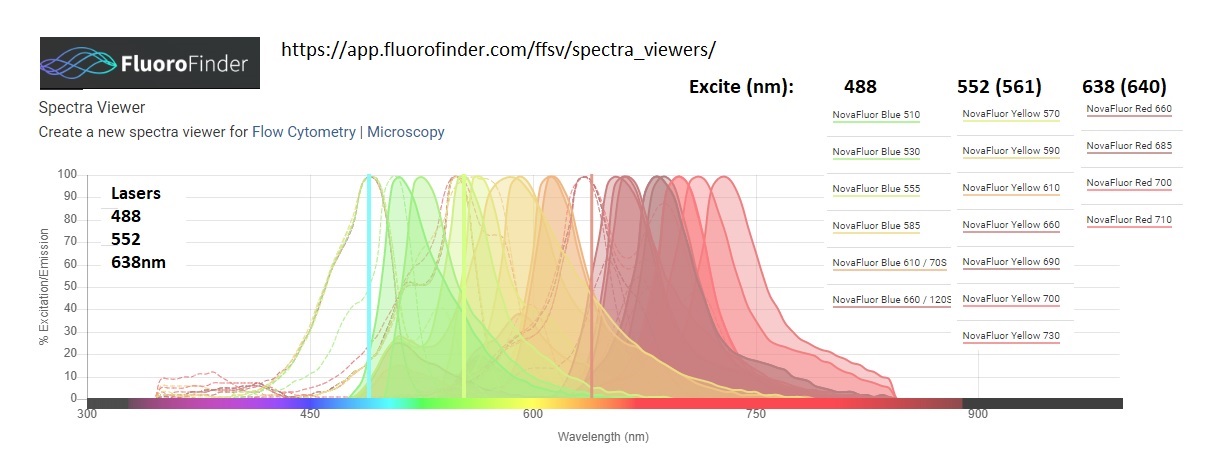
Spectra below from FluoroFinder (17plex, ignoring 2 dim) added 20220126W -- First Nova Color [Blue, Yellow, Red] are the "base dye", rest of color are tandem FRET donor (base dye) --> acceptor(s). The "phiton" technology is a spinout of a Duke University lab, using clever DNA structure (DNA origami) that looks like a "+" (four arms) with dye(s) positioned inside to protect from environment (re: O2 and other quenchers) and to space and orient dyes to each other to optimize tandems (ex: position dyes end to end or in parallel, with specific spacing, for dipoles to be oriented for optimal FRET (fluorescence resonance energy transfer). 100% FRET efficiency is an ideal way to make efficient use of dyes and spectra: 3 excitation wavelengths enabled 17 fluorophores shown below -- various molecules excite different wavelengths (due to donors) and emit at same wavelengths (enabling no moving parts in front of multiplex detectors). Also looks like the "base" Nova Yellow and Nova Red and tandemized to the base Nova Blue for 2 FRET molecules, and base Nova Yellow is tandemized with the base Nova Red for another tandem.
***
20211117W - more on StreamBio UK CPNs = conjugated nanoparticles ... potentially bright enough to see SINGLE molecules ... iron core to enable being pulled down by magnet and outer bright fluorescent shell.
https://www.streambio.co.uk
https://www.streambio.co.uk/wp-content/uploads/2020/07/CPN_Product-list_ver1.0_jun2020_v2.1_Updated_V2.pdf
Product ex max / em max GM notes - FV3000RS confocal and FISHscope have all necessary ex wavelengths
CPN 420 (Violet) 390 / 420 405nm laser or ~395nm LED
CPN 435 (Indigo) 390 / 435 405nm laser or ~395nm LED
CPN 475 (Blue) 390 / 475 405nm laser or ~395nm LED
CPN 510 (Green) 450 / 510 ~440nm light source (FV3000RS laser usually off - user can turn on then off)
CPN 550 (Yellow) 470 / 550 ~488nm light source (laser or LED).
CPN 610 (Orange) 480 / 610 ~488nm light source (laser or LED).
CPN 680 (Red) 400 / 680 405nm laser or ~395nm LED ... Long Stokes Shift (LSS)
two NIR CPNs NOT likely to be useful on our fluorescence microscopes:
CPN 900 (IR-I) 650 / 900 Emission "way out" in NIR - not suitable for our microscopes.
CPN 1130 (IR-II) 750 /1130 Emission "way out" in NIR - not suitable for our microscopes.
Thought experiment: the seven usable-on-our-microscopes CPNs could enable "dedensify by multiplexing". for example, if the same primary antibody or nanobosy (or FISH probe set) were labeled with EACH of the seven CPNs, and applied at same density to a specimen, with saturaton binding (and assuming equal affinity and kinetics), the density for each would be 1/7th os a single plex. This could facilitate single molecule separation -- maybe not perfect but a lot faster (7 exposures) than a usual STORM single molecule localization experiment. With the right microscope (ex 405 & 440 & 488 nm excitation, 8 cameras of optimzed emission wavelenths [enable some other fluorophore to be imaged]) could acquire one exposure (and then do Z-series, tile scan, etc). Similar logic would apply to Brilliant Violets, Brilliant Ultraviolets, ThermoFisher/Phitonix NovaFluors etc.
***
Bio-Rad 20211118Thur ... 3 excitation wavelengths, potentially 8+9+7 = 24plex (ignoring potential to spectrally -- or multichannels -- resolve with Brilliants, SuperBrights, Nova Fluors, conventional fluorophores).
StarBright Ultraviolet (SBUV) 8plex ... max excitation ~340nm (so may work on FISHscope, 368nm excitation; not on confocals 405nm excitation). Shpuld bne excitable by 2-photon (~700nm) and/or 3-photon (~1000nm) light.
https://www.bio-rad-antibodies.com/flow-cytometry-starbright-ultraviolet-dyes.html
StarBright Dye Max Ex, nm Max Em, nm Brightness (1–5) Comparison Dye
StarBright UltraViolet 400 Dye 335 394 3 BUV395 = Brilliant Ultraviolet 395
StarBright UltraViolet 445 Dye 347 440 3 Alexa Fluor 350
StarBright UltraViolet 510 Dye 340 513 3 BUV496
StarBright UltraViolet 575 Dye 340 569 4 BUV563
StarBright UltraViolet 605 Dye 340 609 4 BUV615
StarBright UltraViolet 665 Dye 340 669 4 BUV661
StarBright UltraViolet 740 Dye 344 743 4 BUV737
StarBright UltraViolet 795 Dye 340 792 2 BUV805
Bio-Rad SBUV availableity - 20220330Wed table
Excitable by the 355 nm laser {GM: FISHscope 368nm LED should work}, StarBright UltraViolet (SBUV) Dyes
https://www.bio-rad-antibodies.com/flow-cytometry-starbright-ultraviolet-dyes.html
Fluorophore ... Availability ... Comparison
(20220330Wed) Fluorophore
StarBright UltraViolet 400 Dye ... now BUV395
StarBright UltraViolet 445 Dye ... soon Alexa Fluor 350
StarBright UltraViolet 510 Dye ... now BUV496
StarBright UltraViolet 575 Dye ... soon BUV563
StarBright UltraViolet 605 Dye ... soon BUV615
StarBright UltraViolet 665 Dye ... now BUV661
StarBright UltraViolet 740 Dye ... soon BUV737
StarBright UltraViolet 795 Dye ... now BUV805
StarBright Violet (SBV) 9plex
https://www.bio-rad-antibodies.com/flow-cytometry-starbrightviolet.html
SBV Dye Range
StarBright Dye Max Ex, nm Max Em, nm Brightness (1–5) Comparison Dye
StarBright Violet 440 Dye 383 436 5 Super Bright (SB) 436 , Pacific Blue, eFluor 450
StarBright Violet 475 Dye 405 479 4 Brilliant Violet (BV) 480
StarBright Violet 515 Dye 402 516 5 BV510, Amethyst Orange
StarBright Violet 570 Dye 402 570 4 BV570
StarBright Violet 610 Dye 402 607 5 BV605, SB600
StarBright Violet 670 Dye 401 667 5 BV650, SB645
StarBright Violet 710 Dye 402 713 5 BV711, SB702
StarBright Violet 760 Dye 403 760 5 BV750
StarBright Violet 790 Dye 402 782 4 BV785, SB780
StarBright Blue (SBB) 7plex
https://www.bio-rad-antibodies.com/flow-cytometry-starbrightblue.html
SBB Dye Range
StarBright Dye Max Ex, nm Max Em, nm Brightness (1-5) Comparison Dye
StarBright Blue 520 Dye TBD TBD TBD FITC, A488, BB515
StarBright Blue 580 Dye 475 580 4 -
StarBright Blue 615 Dye 475 611 3 -
StarBright Blue 675 Dye 474 675 5 PerCP
StarBright Blue 700 Dye 470 705 5 PerCP-Cy5.5, BB700, PerCP-eFluor710
StarBright Blue 765 Dye 474 763 4 -
StarBright Blue 810 Dye 475 808 3 -
20230207U: see https://www.bio-rad-antibodies.com/flow-cytometry-starbright-dyes.html for newer StarBright's, including StarBright Yellow (s), StarBright Red (s).
https://www.bio-rad-antibodies.com/flow-cytometry-starbright-dyes.html
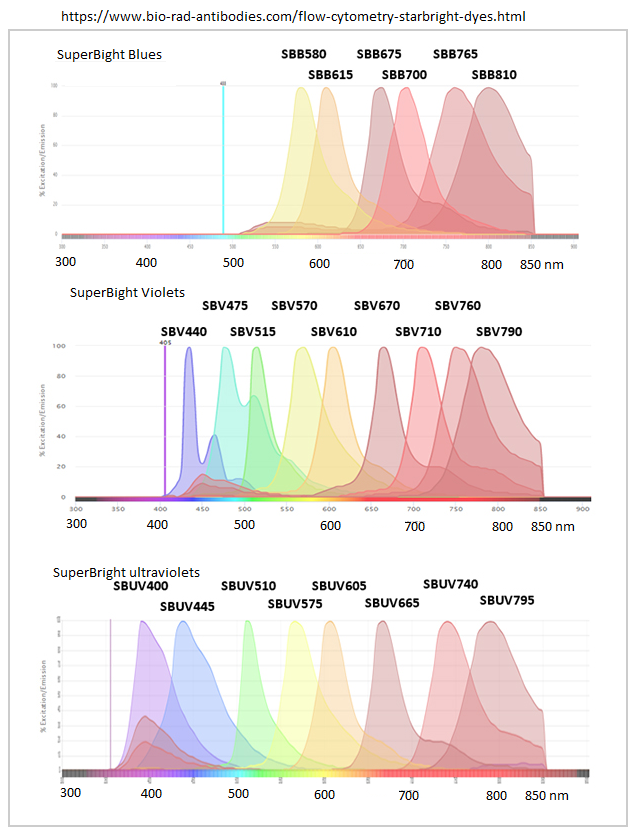
Bio-Rad 202202 StarBright fluorophores emission spectra 488, 405, 355nm excitation laser lines (368nm on FISHscope should excite SBUV's) (gm light edits from their web site)
GM note: ThermoFisher has SuperBrights = Super Brights, ex. https://www.thermofisher.com/us/en/home/life-science/cell-analysis/fluorophores/super-bright-600.html
Graph below top left corner is labeled SuperBright (by me?) but the web address is sclearly Bio-Rad StarBright - my bad - this web site difficult to tweak, so leaving as is with this note.
BioRad posted online potentially useful pdf:
|
Antigen Density for Human and Murine Surface Markers
|




