Zeiss Axio Observer.A1 Inverted Microscope (S972) Use iLab
Inverted fluorescent microscope, Olympus DP80 RGB color and NIR capable monochrome camera
Location: S972
Zeiss Axio Observer.A1 Inverted Microscope in Ross S972 has an Olympus DP80 RGB color + monochrome camera, capable of both RGB histology images and fluorescence. Phase contrast is available for several objective lenses.
***
20191104M (November 4, 2019): we are now on JHU iLab for scheduling.
Ross Imaging Center
If a user needs their PI or administrrtor to set up account numbers (IO#'s in JHU accounting jargon), see
https://help.ilab.agilent.com/36900-managing-your-group/279959-membership-requests-fund-numbers
***
Quck tip, Feb 20, 2019: Optimizing for brightfield image capture, i.e. H&E or IHC (DAB&H):
|
Big News January 11, 2019: We replaced the DP72 RGB camera with Olympus DP80 RGB + monochrome camera. The two CCD sensors are controlled by the same two CellSens buttons for RGB and monochrome, but now moves a hardware component to switch between the two CCD sensors. This will enable much better imaging of Cy5 and Alexa Fluor 647 and other near infrared (NIR) fluorophores. The DP80 RGB color sensor is identical to the DP72. Please note that currently the eyepieces and DP80 are not parfocal - we recommend spending as much time as possible "live" with the DP80 and little time looking through the eyepieces. We are confident the benefits of having RGB + monochrome CCD sensor options outweight the minor inconvenience of not being parfocal. GM thanks John Gibas (Olympus) and Bill Griffith, (rgriff49@jhmi.edu), Sr. Instrument Designer, BME Machine shop, Traylor 4th floor, for big help.
Jan 14, 2019 note: we will probably remove the Eppendorf micromanipulator from this microscope for lack of use. It will remain close by and can be set up again. Users interested in it will need to plan ahead and contact George to see about getting it set up on the "A1".
Suitable specimens include:
* microscope slides ... histology and/or fluorescence (DAPI, green, red, the microscope has a Cy5 filter set - please use the DP80's monochrome camera for single channel fluorescence.
* multiwell plates.
* 35 mm or 60 mm plastic (modest resolution and fluorescence sensitivity) and glass bottom (i.e. Mattek.com P35 series imaging dishes).
Our CellSens 2.02 license includes:
MIA = Multi Image Alignment Algorithm ... panorama
EFI = Extended Focal Imaging
for summary, see brochure at https://unicam.hu/files/olympus-cellsens/cellSens_Attekintes.pdf (PDF also on our server and on acquisition PC).
Olympus CellSens tutorials (videos) online at https://www.olympus-lifescience.com/en/software/cellsens/?utm_content=sf92551011&utm_medium=social-media&utm_source=googleplus&utm_campaign=Olympus%20Life%20Science&sf92551011=1#!cms[tab]=%2Fsoftware%2Fcellsens%2Fresources
***
instrument info
GM note: Zeiss-inverted has a .035 NA long working distance condenser. Our FISHscope has a 0.55 NA long working distance condenser, so in theory could acquire brightfield transmitted light images with 'somewhat higher' spatial resolution. In practice, (i) users not likely to see much benefit [we are happy to try, but recommend moving to fluorescence), and (ii) FISHscope uses a monochrome camera and emission filter wheel (RGB and more!) so a bit unusual to deal with compared to DP80 in color sensor mode.
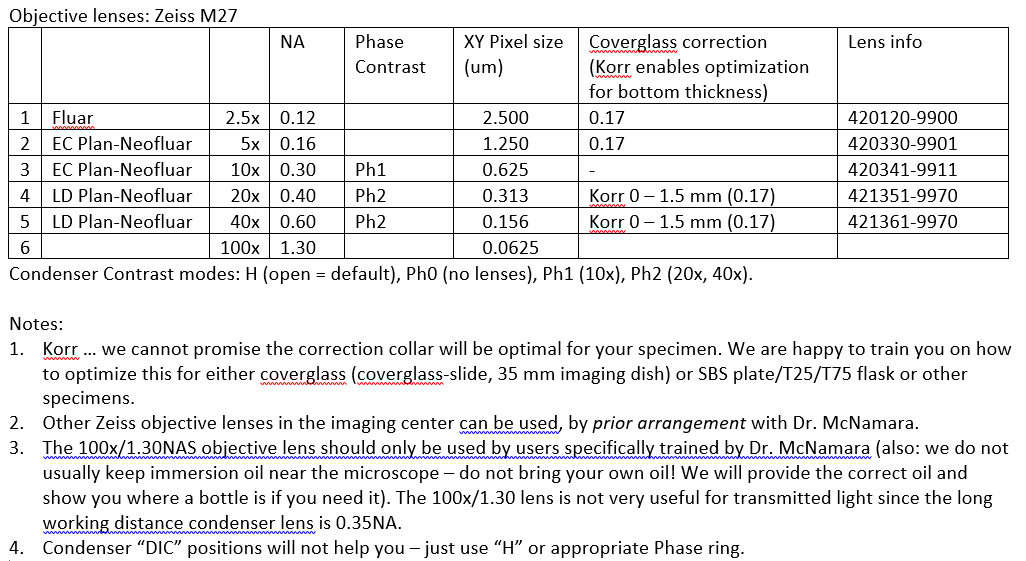
McNamara 20190517Fri – Zeiss AxioObserver A1 filter sets
Zeiss ## filter set specifications are from https://www.micro-shop.zeiss.com/en/de/shop/filterAssistant/filtersets
| Zeiss | Exciter | Dichroic | Emitter | Zeiss catalog | |
| B | 49 | G 365 | FT 395 | BP 445/50 | 488049-9901-000 |
| G | 38 | BP 470/40 | FT 495 | BP 525/50 | 000000-1031-346 |
| R | 43 | BP 545/25 | FT 570 | BP 605/70 | 000000-1114-101 |
| RGB | 25 |
TBP 400 + 495 + 570 |
TFT 410 + 505 + 585 | TBP 460 + 530 + 625 | 488025-0000-000 |
| Cy5 | - |
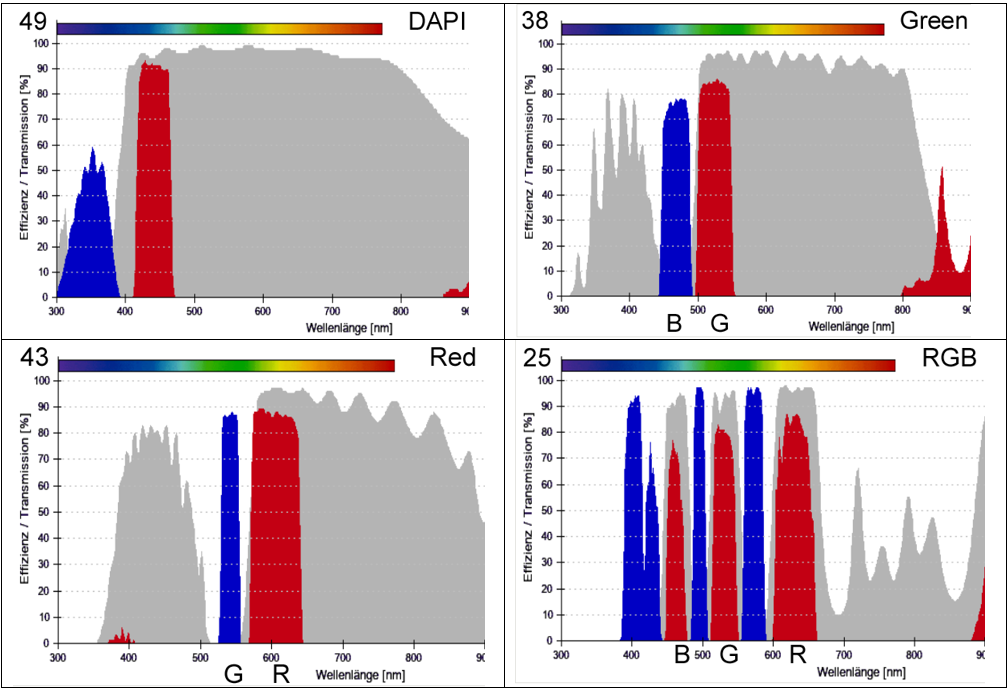
Our Olympus DP80 camera has dual RGB and monochrome CCD sensors (Cellsens acquisition has a toggle for RGB and monochrome). The monochrome sensor is much higher sensitivity, including for Cy5, so should be used for fluorescence. That makes the “RGB” filter set only useful for you to look by eye. The DP80 RGB sensor is ideal for H&E, H&DAB, and similar transmitted light applications (empty cube position between Cy5 and DAPI cubes, or RRGB cube – your choice).
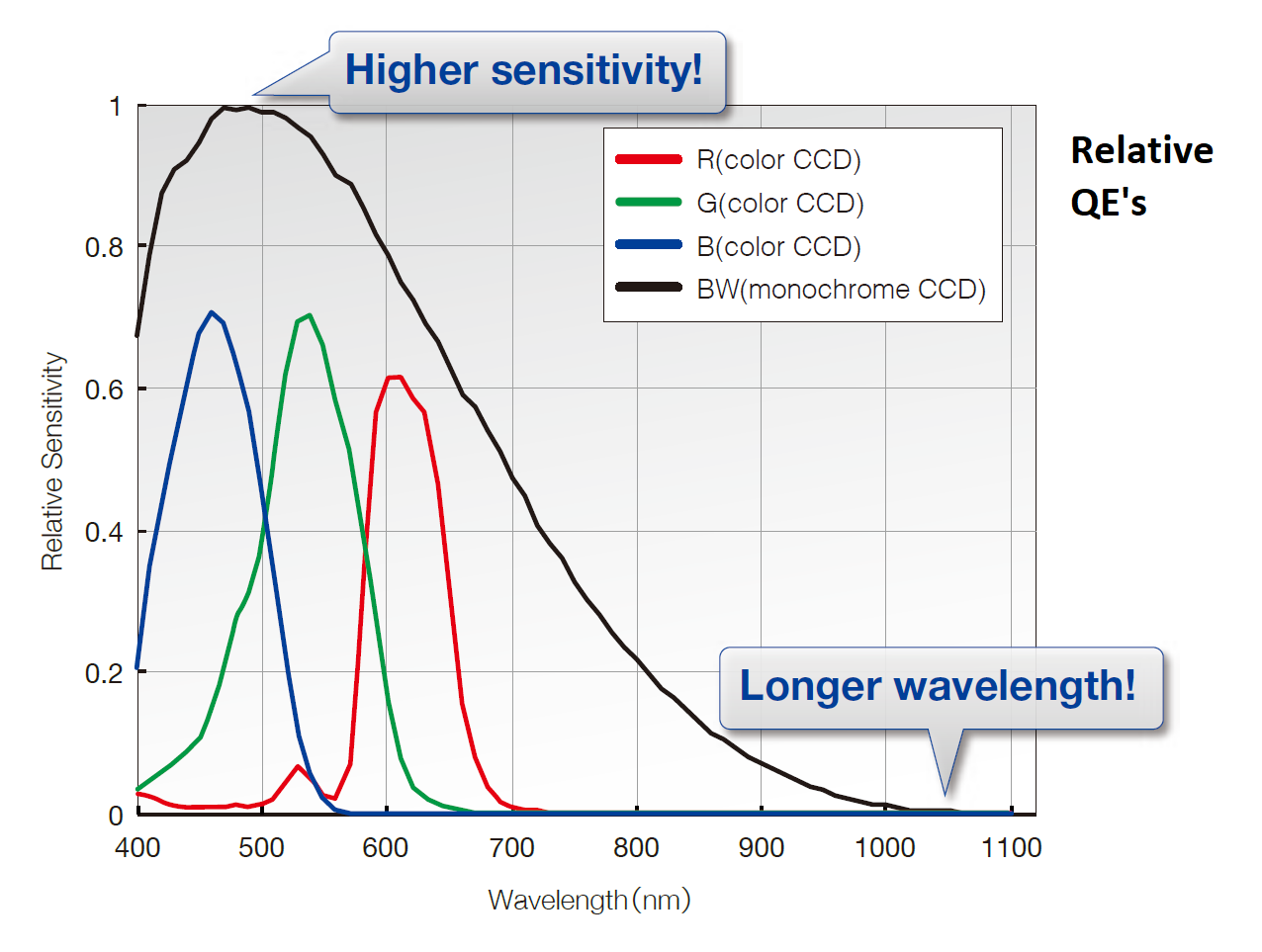
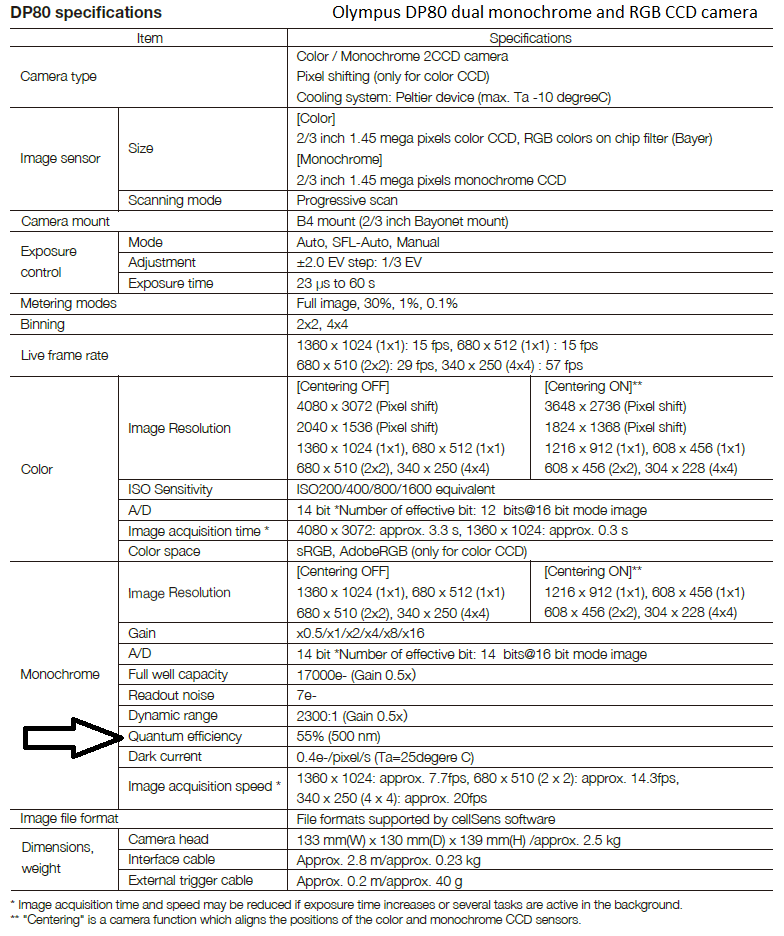
Olympus DP80 dual CCD camera (operated by Olympus cellSens) relative quantum efficiency curves (rQE) ... I note real peak QE of monochrome sensor is 55% (see specifications table), graph is from marketing brochure, so "rQE".
********************************************************************************************************************************************************************************************
|
20200629Mon: widefield brightfield microscopy vs widefield fluorescence microscopy vs confocal fluorescence microscope resulution Ideally, in 2020, no one should need to sit down at a brightfield transmitted light microscope and acquire histology or immunohistochemistry images. The JHU pathology core that has a high resolution transmitted light digital slide scanner, aka whole slide imager (DSI, WSI), to image your slides as a service without training (and with reasonable pricing and turn-around time). For one example, see https://tmalab.jhmi.edu/scanning.html (I do not have an exhaustive list of core facilities or scanners on campus). If you would like to donate a working good quality slide scanner, ideally brightfield and fluorescence (ex. Olympus VS-200 or Leica Biosystems/Aperio scanner), we would love to have it (require approval of image core Director Bin Wu and NIDDK P30 Center Director Mark Donowitz). Vendors: we are happy to host demos, currently (6/2020) no fundign to uy a scanner. Our core does not have any microscopes set up for “high resolution” transmitted light (brightfield) image acquisition. We might be able to get you “good enough” image quality – will need to wait until phase 2 re-opening (I could acquire a few images for you so you can see what we can do with our current equipment). Please note: “high resolution” implies working at or near the optical limit of the wavelength of light and high quality optics. In practice, this usually not needed for immunohistochemistry (ESR1 detected by DAB “brown” IHC using HRP). You have access to the Leica SP8 confocal microscope, whose fluorescence images can operate at “full resolution”. I also note that tyramide signal amplification (TSA) with, say, Alexa Fluor 488 tyramide, can be used as a direct replacement for DAB as HRP substrates (co-substrate being H2O2). The usual microscope for this is our Zeiss AxioImager A1 inverted microscope with DP80 color & monochrome camera (dual CCD). http://confocal.jhu.edu/current-equipment/zeiss-invert-scope-w-olympus-camera (this web page, so did not enable link). It has a long working distance condenser, whose numerical aperture (NA) is 0.35, so will not produce “very high resolution” images”. This is because for brightfield (aka “transmitted”) light, the resolution (dxy) is based on this equation (I’m using “green light 500 nm and 1.3NA of the oil immersion objective lens on our microscope): 1.22 * wavelength 1.22 * 500nm Dxy = -------------------------------------- = ------------------- = 370 nm = 0.370 um NAcondenser + NAobjective 0.35 + 1.3 --> the main reason for long working distance (LWD) condenseris to enable this and similar microscopes to be used for SBS plates, T25 and T75 flasks, and petri dishes. We have confocal micoscopes (and FISHscope) for high resolution imaging, usually by fluorescence. If someone needs high resolution, I recommend microscope slide or 35 mm imaging dish (ex. mattek.com, https://www.mattek.com/store-category/35mm-dishes (the glass bottom is the coverglass, no need to seal the specimen ... which enables iterative rounds of label-image-"de-label", if desired). As noted above, our FISHscope has a 0.55NA long working distance condenser, though practical users are unlikely to see benefits of FISHscope for transmitted light. For comparison, same objective lens in epi-illumination fluorescence: 1.22 * wavelength 1.22 * 500nm Dxy = -------------------------------------- = ------------------- = 235 nm = 0.235 um NAcondenser + NAobjective 1.3 + 1.3
Our confocal microscopes (Leica SP8 and Olympus FV3000RS) have similar NA objective lenses, for Leica SP8 (plan apo 60x / 1.4NA, and replacing the 1.22 (which is 0.61+0.61) in numerator with confocal pinhole 1.0 Airy Units value of 0.51, and simplifying denominator since same lens in and out: 0.51 * wavelength 0.51 * 500nm Dxy (confocal 1.0 Airy unit) = -------------------------------- = ------------------- = 182 nm = 0.182 um NA 1.4
Jeff Reece (NIH) wrote (10/2019 confocal listserv, see below) wrote “When there is plenty of signal (most fixed specimens), I recommend reducing pinhole for better resolution, with a practical limit of roughly 0.5AU. The sweet spot is usually 0.6-0.7AU.” … I am not a fan of ranges, so I simplify this to the “confocal sweetest spot” 0.666 Airy Unit, which can result in ~10% improvement in resolution (0.46 Airy Unit), and note that Brilliant Violet 421 (BV421) emission maximum is 421 nm (in practice, would use 415-455 nm so will use 420nm below), 0.46 * wavelength 0.46 * 420nm Dxy (confocal 0.666 Airy unit) = --------------------------------- = ------------------- = 138 nm = 0.138 um NA 1.4 I note that all the dxy are resolution distances, and that pixel size needs to be smaller to enable Nyquist sampling (see Wikipedia for who Nyquist is and why sample); for a sine wave, one needs to sample 2.2 data points per wave; I recommend 3x sampling for biological images, that is, pixel size = Dxy / 3. For point scanning confocal microscopes like our Leica SP8 and Olympus FV3000RS, pixel size can be optimized by image dimensions (ex. 4096 x 4096 pixels) and zoom, for any given objective lens, and the wavelength of fluorescence emission. Slightly higher resolution can be obtained using reflected light (405 nm laser, 405 nm detection), for example, Cox-Golgi ‘stained’ neurons (see Sivaguru 2019 J Microscopy, for example, with small confocal pinhole).
|
Reserve Equipment


